Mechanisms and Kinetics of Methane Thermal Conversion in a Syngas
fr:Mechanisms and Kinetics of Methane Thermal Conversion in a Syngas
| Ind. Eng. Chem. Res. | |
|---|---|
This paper has been accepted in Ind. Eng. Chem. Res. 2009, 48, 6564–6572.
|
| Test math formulas | |
|---|---|
This paper is used as a first sample in handling formulas.
|
| Wicri network | |
|---|---|
This paper is also available on wiki DCPR.
|
| Anthony Dufour,i anthony.dufour@yahoo.fr |
Sylvie Valin,ii |
| Pierre Castelli,ii | Sébastien Thiery,ii |
| Guillaume Boissonnet,ii | André Zoulalian,iii |
| Pierre-Alexandre Glaude.iv |
- i - Research & Development Division, Gaz de France, 361 avenue du Président Wilson, BP 33, F-93211 Saint-Denis-la-Plaine cedex, France
- ii - CEA, DEN, 17 rue des Martyrs, F-38054 Grenoble, France,
- iii - LERMaB, Nancy-Université, INRA, Faculté des Sciences & Techniques, BP 239, F-54506 Vandoeuvre lès Nancy cedex, France.
- iv - DCPR, Nancy-Université, CNRS, 1 rue Grandville, B.P. 20451 F-54001 Nancy cedex, France..
- Abstract
- In order to optimize H2 and CO production from biomass gasification, the thermal decomposition of methane in a reconstituted syngas was investigated in a tubular reactor at 130 kPa, for a gas residence time of 2 s and as a function of temperature (1000-1400 °C), CH4 (7, 14%), H2 (16, 32%), and H2O (15, 25, 30%) initial mole fractions. H2 showed an inhibiting effect on CH4 conversion whereas H2O had few effects. Three detailed elementary mechanisms were used to predict the methane conversion rate and to identify the key reaction pathways. Flow rate analyses showed that carbon oxidation occurs mainly by addition of OH radicals on C2 compounds. OH radicals are mainly produced by CO2 (CO2 + H = CO + OH). The inhibiting role of H2 on CH4 conversion is explained by a competition between the OH radicals consumption channels (H2 + OH = H2O + H). The competition between thermal conversion of methane and reforming of unsaturated C2 explains the soot formation.
Contents
- 1 Introduction
- 2 2. Materials and Method
- 3 3. Experimental Results
- 4 4. Kinetics of the Apparent CH4 Decomposition Reaction
- 5 5. Simulation of CH4 Decomposition with Detailed Mechanisms
- 6 6. Discussion
- 7 7. Conclusion
- 8 References
Introduction
Biomass gasification is a promising route of gaseous and liquid fuels production such as CH4, H2, Fischer-Tropsch diesel, or methanol. Biomass gasification processes produce a syngas mainly composed of CO, H2, H2O, CO2, CH4, and C2 compounds and tar. CH4 conversion into H2 and CO has been identified as one of the main key points to optimize H2 recovery and the overall energetic efficiency of the H2 production from biomass gasification.1,2 Methane is formed during biomass pyrolysis and tar cracking, and it roughly represents 30 mol % of the hydrogen element contained in the products of wood pyrolysis at 800 °C (including permanent gases, char, water, and tar).3
The most studied methane up-grading technology is catalytic gas conditioning. Metal based catalysts and especially Ni-based catalysts have been extensively studied 4-6. However, they are prone to deactivation due to coke accumulation on the catalytic surface and to sulphur poisoning, when used to up-grade a real biomass syngas 7-9. Other alternative strategies for methane reforming could be the use of carbon-based catalysts such as wood char 10 or the thermal decomposition (without any catalysts).
Thermal decomposition of methane was reviewed by Khan et al. 11, Back and Back 12 and Billaud et al. 13. Pyrolysis of pure CH4 (diluted in an inert gas) was extensively studied in a wide range of conditions 14-26. However, these works focused most of the time on C2H2 production 17, 18, 20, 21, 23, 25 or on carbon vapour deposition 19, 22.
Numerous studies dealt with the effect of H2 15, 17, 18, 23-26 or H2O 17, 24 addition on CH4 thermal decomposition mainly in order to maximise the C2H2 selectivity. The effect of CO2 addition was studied, to our knowledge, exclusively in the presence of O2 27, 28. Moreover, numerous detailed mechanisms validated for an extensive range of CH4 combustion conditions were developed 29-35.
The conversion of CH4 in presence of H2, CO2, H2O, CO with a composition representative of a biomass pyrolysis gas has only been studied, to our knowledge, by Jonsson 36. Jonsson investigated thermal reactions at temperatures lower than 1250∞C. Methane conversions lower than 60% were achieved and no kinetic data were derived.
To our knowledge, in spite of the numerous studies on CH4 conversion, no work deals with the kinetic of CH4 thermal decomposition under the conditions of a biomass pyrolysis gas, i.e. a syngas with a high content of H2, H2O, CO2 and CO. This paper provides new kinetic data on the thermal decomposition of CH4 in such a syngas. The effects of temperature, CH4, H2O and H2 initial mole fractions on CH4 conversion are analysed. The main elementary reactions involved during the syngas thermal up-grading are described and discussed in order to determine the most efficient process conditions and to better understand the limitations, such as soot formation.
2. Materials and Method
The experimental apparatus was formerly presented by Valin et al. 37 with a detailed description on the facility and reactor design. Only the salient points are summarized here.
The syngas composition was controlled by using mass flow rate regulators (Brooks Instrument) for each individual compounds. Water was vaporized in a coiled tube before being mixed with permanent gases in a preheated zone. The nominal molar gas composition was: CH4 7%, CO 19%, CO2 14%, H2 16%, H2O 25%, N2/Ar 7%. When the partial pressure of a compound was modified, the partial pressures of other gases were kept constant by adjusting the nitrogen flow.
The preheated gas was injected in an alumina tubular reactor (838mm length and 70mm diameter). The reactor was specifically designed to study the reforming of hydrocarbons such as methane and tar at high temperatures between 1000 and 1500∞C 37. The absolute pressure in the reactor was approximately 130kPa to make up for the pressure drops, the outlet of the reactor being at atmospheric pressure. Temperature was monitored by 2 type B thermocouples. The gas residence time distribution in the tubular reactor was characterized by unsteady 3D CFD computations by using the Fluent code 37. The 3D simulations were performed under the laminar assumption (Remax=900). The axial dispersion coefficient was determined by using oxygen as a tracer in the simulations 37. The Peclet number is close to 40 for our reactor, which is very close to the plug-flow assumption condition (Pe>50). Moreover, gas composition evolution at the outlet of the reactor was simulated and compared for 3D (laminar assumption) and 1D (plug-flow assumption) simulations, with the same chemical mechanism. The differences between the 2 simulated gas compositions are very small from 0.2s gas residence time 37. Consequently, the plug-flow assumption is justified for our experimental conditions. Nevertheless, an uncertainty of the residence time remains. The relative errors of gas residence time measurements were estimated at ± 0.1s 37. The sensitivity of our results on the uncertainty of the residence time is evaluated in section 5.2.1. The residence time was kept constant as the reactor temperature was changed by adjusting the total flow rate at the entrance of the reactor.
The accuracy of temperature was previously discussed 37. The relative errors of temperature were estimated at ± 15∞C. The relative errors of the gas fractions were estimated at ± 5% except for H2 and H2O. The relative error of H2O lies between -10% and 0%. The absolute error of the H2 mole fraction analysis lies between -2% and 0%. These errors were justified in 37.
At the reactor outlet, soot was separated from the gas phase by 4 filters heated at 200°C. Water was condensed in two cold traps (20°C and 0°C) and continuously quantified.
The dry gas mass flow rate was measured by a Coriolis flow meter (Brooks).
The gas composition was then analysed with a micro gas chromatograph equipped with 3 columns (two 5A molecular sieves, with argon and helium as different carrier gas and one Poraplot Q with helium as carrier gas) and 3 thermal conductivity detectors (Varian).
The analytical system thus allows the determination of the molar flow rate of water and gaseous products. For most tests, more than 94% of the carbon was measured in the gas at the exit 37.
The conversion (Xi) or production (Yi) degree of a compound, i, was then defined as:
The conversion (Xi) or production (Yi) degree of a compound, i, was then defined as
| (1) |
| (2) |
with Fi, the molar flow rate (mol s-1) at the inlet (Fi,in) or at the outlet (Fi,out) of the reactor.
The experimental conditions are given in Table 1.
| Table 1. Experimental Conditions | |||||
|---|---|---|---|---|---|
| mole fraction (%) (balance N2/Ar) | |||||
| temperature (°C) | CH4 | H2O | H2 | CO | CO2 |
| 992-1372 (1187) | 7-14 (7) | 15-30 (25) | 16-32 (16) | 19 | 14 |
| Residence time τ ) 2 s, total pressure 130 kPa, standard conditions in parentheses. | |||||
3. Experimental Results
3.1. Comparison between Experimental Gas Composition and Theoretical Composition at Thermodynamic Equilibrium.
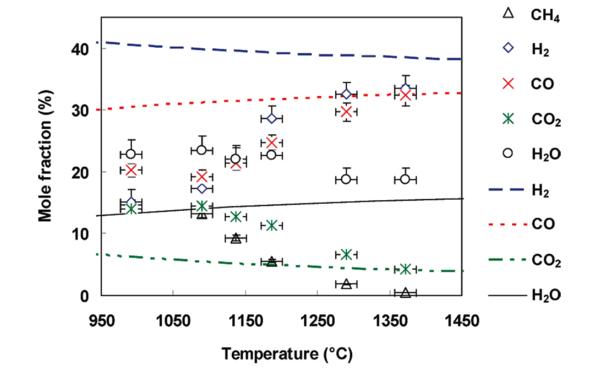
It is of great interest to compare the experimental gas compositions to ones at thermodynamic equilibrium in order to understand how either the thermodynamic equilibrium or the kinetic rates control the gas phase chemistry. The evolution of gas composition at the reactor outlet and at thermodynamic equilibrium (determined with the Gaseq equilibrium program38) is presented in Figure 1.
With increasing temperature, the experimental H2 and CO mole fractions increase whereas the H2O, CO2 and CH4 mole fractions decrease. A methane conversion close to 100% is obtained at 1370 °C. The H2O mole fraction decreases less than the CO2 one (see figure 1). At 1372 °C, a H2 mole fraction of 25 and 33 % was achieved for an initial CH4 mole fraction of 7 % (results not shown) and 14 %, respectively. Carbon atoms from CH4 are mainly converted to CO. The H2/CO ratio is close to 1 for both CH4 mole fractions.
The CH4 mole fraction at equilibrium is not represented in figure 1 because calculations predict a CH4 mole fraction below 1ppmv for all temperatures. The experimental CO and CO2 mole fractions are close to the one determined at thermodynamic equilibrium above 1300 °C. H2 and H2O do not seem to reach thermodynamic equilibrium below 1400 °C. This former observation and the detection of CH4 and soot at the outlet of the reactor evidence that the reactive system does not reach thermodynamic equilibrium in our conditions. Consequently, a kinetic approach is suitable to describe the evolution of the gas composition, even at the highest temperatures.
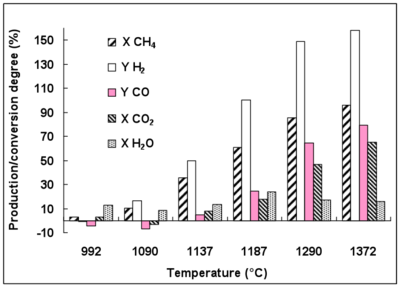
3.2. Conversion and Production Degrees.
A significant H2 production rate of 150% is achieved at 1370 °C (Figure 2). H2 is mainly produced from CH4 conversion and only a small part of H2 comes from H2O conversion. Indeed, little H2O conversion is observed even at 1290 °C (conversion rate below 25% at 1187 °C). CO2 conversion reaches 65% at 1370 °C. The detailed reactions which are involved in these gas conversions are discussed later.
3.3. Experimental Results of CH4 Conversion.
3.3.1. Effect of Temperature and Initial CH4 Mole Fraction.
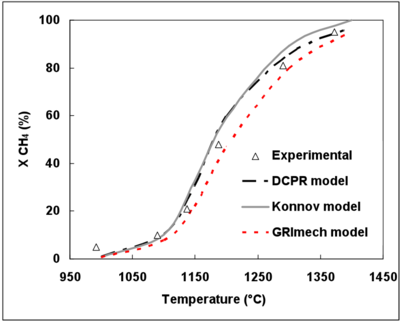
CH4 conversion runs from 5% at 992°C to 95% at 1372°C (Figure 3). The initial CH4 mole fraction has a sensitive effect for its conversion, between 1137 and 1290°C. At 1137°C, CH4 conversion is 21% and 36% for 7%mol. and 14%mol. CH4initial mole fractions, respectively.
The determined CH4 conversion cannot be compared with kinetic data from the literature as no reference, to the our knowledge, dealt with CH4 conversion in similar conditions (temperature, initial gas composition and gas residence time) than ours. The experimental CH4 conversion rates are more extensively discussed later and compared to the predictions of detailed mechanisms (section 5.).
| Figure 3. Effect of temperature and initial CH4 mole fraction on CH4 conversion (t=2s) initial CH4 mole fraction of 7% (a) and 14% (b). |
3.3.2. Effect of H2 and H2O on CH4 Conversion.
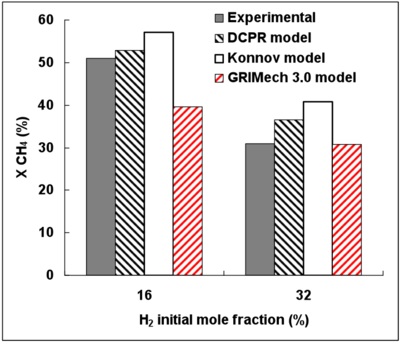
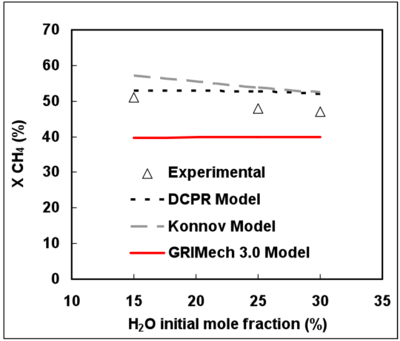
Figure 4 presents the effect of H2 initial mole fraction on CH4 conversion. Experimental results put in evidence the inhibiting effect of H2 on CH4 conversion. This finding is in agreement with many former works 15, 18, 36. As the H2 initial mole fraction increases from 16 to 32%vol., the experimental CH4 conversion rate decreases from about 50% to 30%.
From 15 to 30% initial mole fraction, H2O has only a slight chemical effect on CH4 conversion at 1187°C and for a gas residence time of 2s (see Figure 5). Moreover, a slight inhibiting effect of H2O on CH4 conversion can be noticed.
4. Kinetics of the Apparent CH4 Decomposition Reaction
The apparent CH4 decomposition rate in the reactor, rCH4, was described by the following equation:
| (3) |
- with:
- rCH4 (mole m-3 s-1), the reaction rate of CH4 decomposition,
- Pi (atm.), the average partial pressure of each compound, i, between the inlet and the outlet of the reactor,
- m, n and p, the apparent reaction order of CH4, H2, and H2O respectively,
- k (mole m-3 s-1 atm-(m+n+p)), the apparent rate constant of CH4 decomposition.
The rate constant of CH4 decomposition was assumed to follow an Arrhenius law, as:
- with:
- ko (mole m-3 s-1 atm-(m+n+p)), the pre-exponential factor,
- E (J mol-1), the activation energy,
- R, the universal gas constant (R=8.314 J mol-1),
- T (K), the temperature.
The CH4 concentration profile along the reactor length was not known. Consequently, the CH4 decomposition rate could not be integrated for each elemental volume of the reactor. An average CH4 decomposition rate was calculated, as:
| (4) |
- with:
- P0 CH4 (atm.), the CH4 partial pressure at the inlet of the reactor,
- X, the CH4 conversion rate,
- R, the universal gas constant (8,2.10-5 m3.atm.mol-1.K-1),
- T (K), the reactor temperature (K),
- τ (s), the gas residence time.
The calculation is only valid for a tubular reactor, for the given residence time (2s) and range of temperature. The reaction orders m, n, and p, were determined from equation (3) by systematic variation of one of the CH4, H2 or H2O partial pressures at 1187°C (experimental results presented in figure 3, 4 and 5; the partial pressures of other gases were kept invariable by adjusting the nitrogen flow). The reaction orders were : m=1.48, n= -0.91, n= -0.11.
The rate constant, k (mole m-3 s-1 atm-0,46), can then be calculated, for each experimental point, as:
| (5) |
The activation energy and pre-exponential factor were determined from experiments at different temperatures (figure 3).
The CH4 decomposition rate can be described for our experimental conditions (summarized in table 1) by the following relationship:
The value of the activation energy (211 kJ mol-1) is lower than the one determined for instance by Bruggert et al. 22 or Holmen et al. 23 (400 kJ.mol-1) for the decomposition of pure CH4, with a different gas composition and lower CH4 conversion rates than in our case.
Due to higher CH4 conversion rates investigated in this work, the initiation reactions, which are characterized by a higher activation energy, become minor reactions whereas the propagation reactions decrease the apparent activation energy. Moreover, a lower apparent activation energy is determined in our case due to the consideration of an average methane conversion. Indeed, the determined conversion rate which is an average along the reactor is lower than the initial value at the entrance of the reactor and especially when the temperature increases with a constant gas residence time. Consequently, the effect of temperature on methane conversion rate and the activation energy is lowered. Moreover, as it will be shown hereafter, H2, H2O, CO and CO2 have a kinetic effect on methane conversion.
5. Simulation of CH4 Decomposition with Detailed Mechanisms
5.1. Presentation of the Mechanisms.
Global kinetic models (as developed in the previous section) are generally only validated for a short range of experimental conditions and do not describe the elementary pathways of CH4 decomposition. To overcome these drawbacks, many detailed mechanisms were developed especially for the combustion of methane 29-35. The aim of this section is not to develop a new mechanism but to compare some previously developed mechanisms and to identify the main elementary reactions and the most sensible ones for CH4 decomposition in our operating conditions.
For this purpose, 3 detailed mechanisms were used and compared: the « DPCR » (mechanism developed at Département de Chimie Physique des Réactions, CNRS, Nancy, France), « Konnov’s » and « GRImech3.0 » mechanisms. These mechanisms were validated for an extensive range of CH4 (and some heavier hydrocarbons as described hereafter) combustion conditions. The sensitive reactions in our experimental conditions will be different to that in combustion due to high concentrations of CO and H2 (reduction conditions) and slow oxidation rates (CO2, H2O). Their kinetic constants may be known less accurately. Nevertheless, these elementary mechanisms could be valid to predict the reactivity of CH4 and some other species in our experimental conditions as well.
The « DCPR » mechanism were previously developed by Barbé et al. 30 and then updated by Fournet et al 39, Belmekki et al 40 and Gueniche et al. 41. The reaction base includes 116 species and 811 elementary reactions. It was validated for the formation and oxidation of CH4, C2H6, C3–C4 unsaturated hydrocarbons and benzene. This mechanism is the only one of the 3 ones investigated in this paper, which takes into account the benzene formation and oxidation.
The « Konnov’s 0.5c » mechanism 34 was validated for the oxidation of light hydrocarbons species from CH4 to C3. It includes 127 species and 1213 elementary reactions. It originates from Borisov et al. 42, 43 and was largely updated on the basis of recommendations of Baulch et al. 44. The « Konnov’s » mechanism is the more systematic one of the 3 mechanisms because it includes all the species to C3 and was validated in the larger range of conditions (see 34 for more details). The updated release 0.6 of the Konnov mechanism includes improved nitrogen chemistry, which is not of importance in the present study. This newer version will not influence the presented predictions.
The « GRImech 3.0 » mechanism was developed by Smith et al. 35 and was optimized for a large range of natural gas combustion conditions (see 35, for more details). It is a compilation of 325 elementary reactions which involve 53 species. This mechanism does not include C3+ species except propane.
Simulations were performed using the SENKIN code from Chemkin II 45, assuming an isothermal plug-flow reactor as justified in a previous work 37. The SENKIN code enables to determine the gas composition evolution along the reactor length as a function of the gas residence time.
5.2. Comparison between experimental and simulated results.
5.2.1. Gas composition
Figure 6 displays the gas composition evolution (except for CH4) simulated by the DCPR mechanism as a function of temperature. The 3 mechanisms (results not shown for the 2 other ones) satisfactorily reproduce the gas composition. The sensitivity of the residence time uncertainty (2s ± 0.1s) on predicted mole fractions was analysed. The variation of predicted gas compositions is lower than 4% of the average outlet mole fraction for all gases and at all temperature in the range of gas residence time. For instance, at 1200°C which the most sensitive temperature for methane conversion, the predicted methane conversion lies between 69.1 and 70.3% in the range of gas residence time uncertainty.
CO2, CO, H2, and H2O mole fractions are well predicted by DCPR mechanism except at 1400°C where the production of H2 and the consumption of H2O are slightly over-predicted. These over-predictions are higher with the Konnov’s mechanism and start from 1300°C (not shown). The Konnov’s mechanism underestimates the CO2 consumption between 1100 and 1300°C. The GRImech mechanism underestimates H2 and CO production and H2O consumption between 1100 and 1300°C. The start of the H2O consumption is predicted by the 3 mechanisms at about 1200°C whereas the start of the CO2 consumption is predicted at about 1100°C.
5.2.2. Effect of temperature and initial CH4 mole fraction.
Figure 3.(a) and (b) display the evolution of the experimental and simulated CH4 conversion degrees as functions of temperature and initial CH4 mole fraction.
The 3 mechanisms exhibit a very good agreement as to the effect of temperature on the CH4 conversion rate.
The DCPR and Konnov’s mechanisms give very close results and the effects of CH4 initial mole fraction and temperature are well predicted. The GRImech mechanism slightly underestimates the CH4 conversion rate with a shift of 10°C and 25°C for an initial CH4 mole fraction of 7% and 14% respectively. This is the mechanism which predicts the least the increase in the CH4 conversion degree when the initial CH4 mole fraction increases.
5.2.3. Effect of Initial H2 and H2O Mole Fraction.
Figure 4 displays the experimental and simulated CH4 conversion degrees as a function the initial H2 mole fraction.
The DCPR and Konnov’s mechanisms predict satisfactorily the effect of H2 on the CH4 conversion. The inhibiting effect of H2 is nevertheless slightly under-estimated.
The GRImech mechanism gives the closest conversion degree prediction for the highest H2 mole fraction (32%) but the H2 inhibiting effect is not well predicted.
Figure 5 shows that the very low effect of H2O on CH4 conversion is well represented by the 3 mechanisms.
6. Discussion
6.1. Flow Rate Analyses.
The flow rate analysis simulated by the 3 mechanisms were compared in order to derive the main reaction pathways of CH4 conversion involved in our experimental conditions and to explain the effects of H2 and H2O initial mole fractions on methane conversion by the mean of elementary mechanisms.
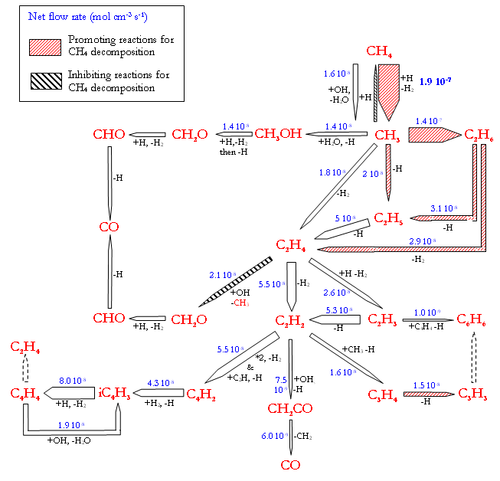
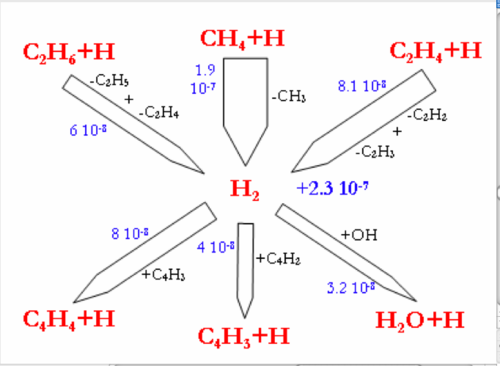
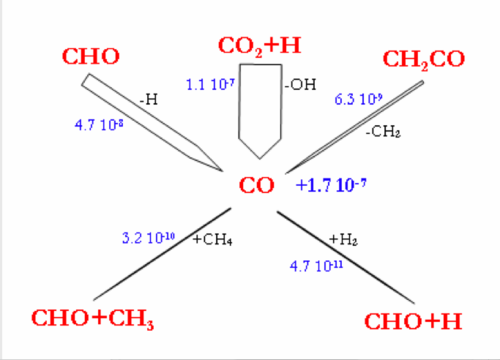
Flow rate analyses were performed at 1200°C and for a gas residence time of 1.1s, corresponding to a CH4 conversion degree of about 30-40%. Under these conditions, the flow rate analyses highlight the intermediate reactions occurring in the CH4 thermal decomposition. Figure 7 and 8 display the reaction flows, and the sensitive reactions in CH4 decomposition and in H2, H2O, and CO production and consumption, taken from the DCPR model. The flow rate analysis performed with the 2 other models (Konnov and GRIMech) are not shown.
| Figure 8. Flow rate analysis of the main reactions of H2O, H2 and CO production and consumption, DCPR model, 1200°C, τ=1,1s Standard initial mole fractions. |
The main pathway of CH4 consumption (DCPR model, figure 7) is the H-abstraction by H atom to give a methyl radical and H2 (CH4+H=CH3+H2). In our conditions of intermediate CH4 conversion, the concentration of H atoms is large and the H-abstraction is more important than the initiation reaction (CH4=CH3+H). This methathesis is favoured by the low enthalpy of reaction. The dissociation energy of the C-H bond (440 kJ/mol) is roughly provided by the energy of the H-H bond formation (436 kJ/mol). The ratio of the H-abstraction of CH4 by OH radicals is less than 10%. Methyl radicals then lead to the formation of ethane (C2H6), which is mainly dehydrogenated into ethene (C2H4) and then acetylene (C2H2). C2H4 and C2H2 are slowly oxidised by OH radicals and lead mainly to propagation reactions involving C2 and C4 species. The oxidation of carbon (formation of a C-O bond) occurs mainly by the addition of OH radicals on C2H4 (2.1 10-8 mol cm-3 s-1) and on C2H2 (7.5 10-9 mol cm-3 s-1). The main reactions of C2H4 are H abstraction by H atoms and OH addition (C2H4+OH=CH3+HCHO). Metatheses by OH on C2H4 (C2H4+OH=C2H3+H2O) are a minor channel compared to OH addition. It represents only 13% of the total rate of these 2 reactions involving OH radicals with C2H4.
The direct oxidation by H2O of CH3 radicals only accounts for 30% (1.4 10-8 mol cm-3 s-1) of the formation of a C-O bond.
Benzene, which is known as a soot precursor, is mainly formed from C3H3 radicals, which are mainly formed by C2H2. The formation of C2H2 (and then benzene and soot) is favoured by the poor direct oxidation of CH3 radicals.
H2O is mainly produced by the reaction (H2+OH=H2O+H) and the reactions of H2O consumption are still slow (figure 8.). H2 is mainly produced by the addition of H radicals on CH4 and by dehydrogenation of C2 species. H2 is mainly consumed by the propagation reactions involving C4 species, according to the DCPR model. CO and OH radicals are mainly produced by the addition of H atom to CO2 (CO2+H=CO+OH) and are consequently not very dependant on H2O concentration underour conditions of high concentration of CO2. About 30% (4.7.10-8 mol cm-3 s-1) of CO is produced from CHO, which is mainly formed by C2H4 and CH3oxidation.
The Konnov’s model (flow rate analysis not shown) exhibits the H-abstraction to yield a methyl radical and H2 (CH4+H=CH3+H2), as the main reaction pathway of CH4 consumption. Methyl radicals then lead to the production of C2H6, to C3 and C4 (as C4H6). C2H6 and C4H6 then lead to the production of C2H2 which is mainly oxidised by OH radicals into CO. H2O is poorly reactive. H2 is mainly produced from CH4 and C2 species. CO is mainly produced by CO2+H and by CHO and CH2CO conversion.
In the GRImech model, for the considered CH4 conversion degree (~30%), the initiation reaction (CH4=CH3+H) still accounts for 37% of the consumption of CH4. The H-abstractions by H atom (CH4+H=CH3+H2) and OH radicals (CH4+OH=CH3+H2O) account for 51% and 11% respectively of the CH4 consumption. The GRImech model mainly exhibits the following consecutive steps, with very minor other channels: CH4 → CH3 → C2H6 → C2H4 → C2H2 → CO. The oxidation solely occurs by the addition of OH radicals onto C2H2. CO is mainly produced by the CO2+H reaction. H2O is not very reactive.
6.2. Comparison of the Flow Rate Analyses and of the Sensitive Reactions
In the 3 models, the initiation reaction is: CH4=CH3+H. Nevertheless, this reaction represents a larger flow in the GRImechmodel due to a lower predicted concentration of radicals. This channel appears less in the DCPR and Konnov’s simulations because a larger concentration of radicals (especially H atoms) is predicted. Consequently, the propagation reactions represent the major flow rate in these 2 models for the investigated conditions.
In the 3 models, the propagation reactions involve methyl radicals which promptly lead to the formation of C2H5/C2H6, then dehydrogenated into C2H4. This reaction pathway is well known for pure methane decomposition 12, 18. The propagation is then different for the 3 models.
The Konnov model exhibits propagation with C3 species, and the DCPR model with C4 species. C3 and C4 species may be overestimated because competing reactions of addition yielding heavier compounds are not included in the models.
The GRImech model exhibits the conversion of C2H4 solely into C2H2. The GRImech model does not predict the formation of species heavier than C2 because it does not include these species (except C3H8 and C3H7). Consequently, an accumulation ofC2H2 is predicted, which is not realistic in pyrolysis conditions.
In the 3 mechanisms, the propagation reactions CO2+H=CO+OH and H2+OH=H2O+H exhibit important flow rates, except the former reaction for the Konnov’s mechanism. The CO2+H reaction has already been identified as the primary step responsible for the chemical effect of CO2 in several previous study 27, 28 under oxy-fuel combustion conditions, but was never identified, to our knowledge, under our conditions of a syngas composition.
The 3 mechanisms show that the oxidation process occurs mainly by the addition of OH radicals onto C2 species. This mechanism is similar to the oxidation of benzene by the breaking of the bond 41. In the 3 mechanisms, minor channels involve the formation of a C-O bond directly from CH4 or CH3 radicals.
In the 3 models, the promoting reactions for CH4 decomposition are the reactions which lead to the formation and the conversion of the CH3 radicals. The reactions of CH3 and C2H6 consumption are very promoting reactions for CH4 conversion in both DCPR and Konnov’s models.
The inhibiting reactions for CH4 conversion are the reactions of OH radicals addition on C2H4 (DCPR model) or on C2H2 (Konnov model). The sensitivity analysis of the GRImech mechanism does not exhibit inhibiting reactions.
6.3. Effect of Initial H2 and H2O Mole Fraction on the Reaction Pathways.
The flow rate analysis were performed for the 3 models at 1200°C, 1.1s gas residence time, and for an initial H2 mole fraction of 32 % (not shown). The effect of H2 on the reaction pathways was studied by the comparison of the flow rate analysis performed for the 2 different H2 initial mole fractions (16%vol., displayed on figure 7 and 8 for the DCPR model, and 32 %vol).
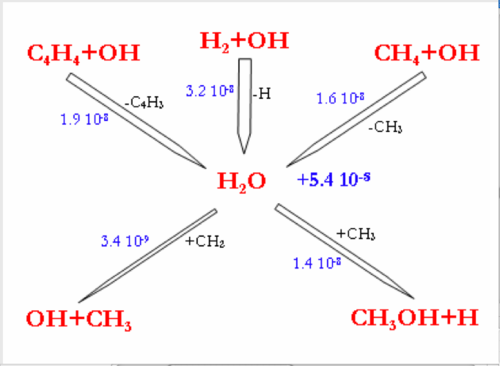
In the 3 mechanisms, at higher H2 mole fraction, the consumption of OH radicals by the H2+OH reaction is favored. OH radicals become less available for the C2 oxidation reactions, which explains the inhibiting effect of H2 on CH4 conversion.
Nevertheless, the flow rate of the (C2H4+OH=CH2O+CH3) reaction (inhibiting reaction in the DCPR mechanism) is increased in the DCPR and Konnov’s mechanisms, because the C2H4 production is increased by the hydrogenation of C2H2 (C2H2+H2=C2H4) when H2 mole fraction increases.
The larger consumption of OH radicals by H2 leads to a large increase in H2O production. H2O production is almost doubled in the DCPR model when H2 initial mole fraction is increased from 16 to 32% and increased by a factor of ten in the Konnov’s model prediction.
In the GRImech mechanism, the OH radicals are mainly consumed by H2. The OH radicals are slowly consumed by the C2 species (accumulation of C2H2). The large decrease of OH radicals due to their almost exclusive consumption by H2 has few effects on the CH4 conversion rate in the GRImech mechanism. The reactions of OH addition onto C2H2 involve very minor channel in all cases, for high or low H2 mole fractions. CH4 conversion rate is thus not sensitive to the modification of this minor channel.
Concerning the effect of initial H2O mole fraction on the CH4 conversion, the 3 mechanisms exhibit about the same predictions (see figure 5). Indeed, the initial H2O mole fraction has few effect on CH4 conversion because the OH radicals are mainly produced at 1200°C by CO2+H due to the high concentration of CO2 under our conditions. OH radicals concentration is thus relatively independent of H2O concentration. Though H2O has very few chemical effects under our conditions, H2O could exhibit experimentally a thermal effect due to its high thermal capacity, thus leading to better isothermal conditions in the reactor and to a lower soot formation.
6.4. Soot Precursors.
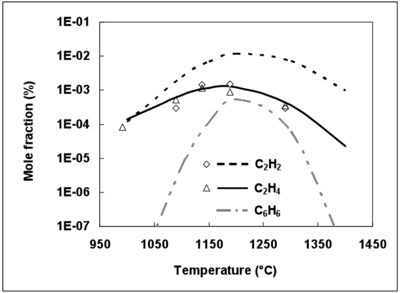
It is now well established that C2H2 is one of the main soot precursors 46,47. The 3 mechanisms predict a maximum mole fraction of C2H2 at 1200°C (not shown), which is in agreement with numerous gas combustion studies 47. It was shown that soot formation could be estimated by monitoring the benzene mole fraction in some premixed flames 48.
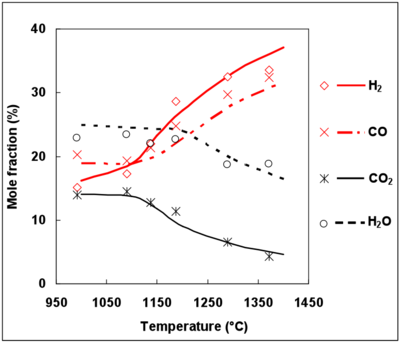
Since the DCPR mechanism includes soot precursor, such as benzene, formation and conversion, C2H2, C2H4 and benzene mole fractions were simulated with this model and compared with experimental data for C2H2 and C2H4 (figure 9). The experimental mole fractions of benzene were unfortunately not available.
The maximum mole fraction for C2H2, C2H4 and C6H6 is obtained for the same temperature (1200°C). The prediction of C2H4 mole fractions is in agreement with the experimental mole fractions, whereas C2H2 mole fractions are overestimated by the DCPR mechanisms (see figure 9). Future works should deal with the improvement of mechanisms for soot precursors predictions under the conditions of a syngas thermal reforming. Benzene and soot are formed by unsaturated compounds and by secondary tar in the case of a real biomass syngas 49, 50. Complementary studies are needed to understand soot formation mechanisms in a real biomass syngas and to precise the conditions which minimize soot formation.
6.5. Effect of C2H4.
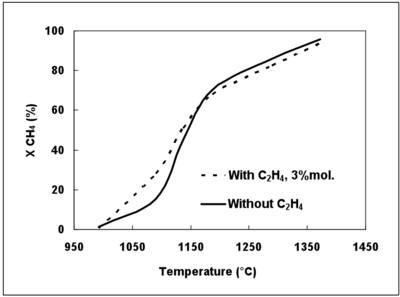
The reconstituted syngas used during this study did not contain tar, C2 or other compounds which are produced by the pyrolysis of solid fuels and which could impact the CH4 conversion. The effect of a compound present in a real syngas was investigated by using C2H4 as a surrogate. Figure 10 displays the effect (simulated with the DCPR mechanism) of C2H4 on the CH4 conversion degree.
The presence of 3%mol. of C2H4 increases the CH4 conversion at temperatures lower than 1150°C. The production of H radicals is favoured by H-abstraction to give C2H3. C2H3 yields another H atom and C2H2, whereas CH3 radicals mainly lead to the formation of C2H6 and of CH4 (CH3+H2 = CH4+H). The addition of C2H4 involves then a larger concentration of H radicals at low temperature which favours the CH4 conversion (CH4+H=CH3+H2) and other propagation reactions. The effect of C2H4 decreases when the H2 initial mole fraction increases (not shown). Indeed, H2 has an inhibiting effect on C2H4 conversion due to a shift of the equilibrium of the C2H2 hydrogenation (C2H2+H2=C2H4).
7. Conclusion
The aim of this work was to determine the kinetics of the thermal decomposition of CH4 in a reconstituted syngas and to understand the elementary pathways.
For this purpose, the thermal decomposition of methane was studied in a tubular reactor at a total pressure of 130kPa, a gas residence time of 2s and as a function of temperature (1000-1400°C), CH4 (7, 14 vol.%), H2 (16, 32 vol. %) and H2O (15, 25, 30 vol. %) initial mole fractions.
It was shown that the global decomposition rate of CH4, r (mol m-3 s-1), can be described by the following expression:
The results clearly indicate that temperatures above 1300°C at a residence time of 2s are necessary to convert more than 80% of methane.
To identify the main elementary reactions of CH4 decomposition in our operating conditions, 3 detailed mechanisms developed of CH4 combustion were used. The 3 mechanisms exhibit good agreementas to the effect of the temperature on the CH4 conversion. The flow rate analyses simulated by the 3 mechanisms were compared in order to derive the main pathways of CH4 conversion involved in our experimental conditions.
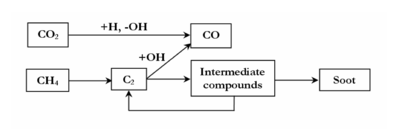
The 3 mechanisms lead to the same overall reaction scheme of thermal conversion of CH4 in the presence of H2, H2O and CO2 (figure 11).
Minor channels involve the “direct” oxidation of CH4 or CH3 radicals in our conditions. The oxidation of carbon occurs mainly by the addition of OH radicals on C2 species. OH radicals are mainly produced by CO2 (CO2 + H = CO + OH). The inhibiting role of H2 on methane decomposition is explained by a competition between the different OH radicals consumption channels (H2+OH=H2O+H). H2O involves few chemical effects due to the high concentration of CO2 which mainly controls the OH radicals production. The effect of C2H4, as a surrogate of heavier hydrocarbons in the syngas, was simulated. The effect on methane conversion and soot formation of other compounds also present in a syngas produced by biomass pyrolysis such as C2, tar, N and S compounds (etc.) has still to be studied.
References
- (1) Spath, P.; Aden, A.; Eggeman, T.; Ringer, M.; Wallace, B.; Jechura J. Biomass to Hydrogen Production Detailed Design and Economics Utilizing the Battelle Columbus Laboratory Indirectly-Heated Gasifier, Technical Report NREL/TP-510-37408, 2005, 161p.
- (2) Papadopoulo, M.; Normand, S.; Dufour, A.; Muller, T.; Evaluation of hydrogen production from biomass gasification, 2nd European Hydrogen Energy Conference, Zaragoza, Spain, 2005.
- (3) Dufour, A.; Girods, P.; Masson, E.; Rogaume, Y.; Zoulalian, A. Synthesis gas production by biomass pyrolysis: effect of reactor temperature on product distribution. Int. J. Hydrogen Energy 2009, 34, 1726.
- (4) Courson, C.; Makaga, E.; Petit, C.; Kiennemann A. Development of Ni catalysts for gas production from biomass gasification. Reactivity in steam- and dry-reforming. Catal. Today 2000, 63, 427.
- (5) Swierczynski, D.; Courson, C.; Bedel, L.; Kiennemann, A.; Guille, J. Characterization of Ni-Fe/MgO/olivine catalyst for fluidized bed steam gasification of biomass, Chem. Mat. 2006, 18, 4025.
- (6) Nishikawa, J.; Nakamura, K.; Asadullah, M.; Miyazawa, T.; Kunimori, K.; Tomishige, K. Catalytic performance of Ni/CeO2/Al2O3 modified with noble metals in steam gasification of biomass, Catal. Today 2008, 131, 146.
- (7) Corella, J.; Orío, A.; Aznar, P. Biomass gasification with air in fluidized bed: Reforming of the gas composition with commercial steam reforming catalysts. Ind. Eng. Chem. Res. 1998, 37, 4617.
- (8) Bain, R.L.; Dayton, D.C.; Carpenter, D.L.; Czernik, S.R.; Feik, C.J.; French, R.J.; Magrini-Bair, K.A.; Phillips, S.D. Evaluation of catalyst deactivation during catalytic steam reforming of biomass-derived syngas. Ind. Eng. Chem. Res. 2005, 44, 7945.
- (9) Torres W., Pansare S.S., Goodwin Jr. J.G., Hot gas removal of tars, ammonia and hydrogen sulfide from biomass gasification gas. Catal. Rev. Sci. Eng. 2007, 49, 407.
- (10) Dufour, A.; Celzard, A.; Ouartassi, B.; Broust, F.; Fierro, V.; Zoulalian, A. Effect of micropores diffusion on kinetics of CH4 decomposition over a wood-derived carbon catalyst. App. Catal. A. 2009, 360, 120.
- (11) Khan, M.S.; Crynes, B.L.; Survey of recent methane pyrolysis literature. Ind. Eng. Chem. 1970, 62, 54.
- (12) Back, M.H.; Back, R.A. Thermal decomposition and reactions of methane. Pyrolysis: theory. Ind. Pract.; Albright, L.F.; Crynes, B.L.: Corcoran, 1983.
- (13) Billaud, F.; Baronnet, F.; Freund, E.; Busson, C.; Weill, J. Thermal decomposition of methane. Revue Inst. Fr. Pétrole 1989, 44, 813.
- (14) Kassel, L.S. The thermal decomposition of methane. J. Am. Chem. Soc. 1932, 54, 3949.
- (15) Germain, J.E. ; Vaniscotte C. Craquage du méthane dans un réacteur tubulaire II. Effets de dilution. Bull Soc. Chim. Fr. 1958, 3, 319.
- (16) Skinner, G.B.; Ruehrwein, R.A. Shock tube studies of the pyrolysis and oxidation of methane. J. Phys. Chem. 1959, 63, 1736.
- (17) Kunugi, T.; Tamura, T.; Naito, T. New acetylene process uses hydrogen dilution. Chem Eng. Progr. 1961, 57, 43.
- (18) Eisenberg, B.; Bliss, H. Kinetics of methane pyrolysis. Chem. Eng. Progr. Symp. Ser. 1967, 72, 3.
- (19) Palmer, H.B.; Lahaye, J.; Hou, K.C. On the kinetics and mechanism of the thermal decomposition of methane in a flow system. J. Phys. Chem. 1968, 72, 348.
- (20) Chen, C.J.; Back, M.H.; Back, R.A. The thermal decomposition of methane. II. Secondary reactions, autocatalysis, and carbon formation; non-Arrhenius behaviour in the reaction of CH3* with ethane. Can. J. Chem. 1976, 54, 3175.
- (21) Billaud, F.; Baronnnet, F.; Guéret, C.P. Thermal coupling of methane in a tubular reactor: parametric study. Ind. Eng. Chem. Res. 1993, 32, 1549.
- (22) Bruggert, M.; Hu, Z.; Huttinger K.J. Chemistry and kinetics of chemical vapor deposition of pyrocarbon VI. influence of temperature using methane as a carbon source. Carbon 1999, 37, 2021.
- (23) Holmen, A.; Rokstad, O. A.; Solbakken, A. High-temperature pyrolysis of hydrocarbons. 1. Methane to acetylene. Ind. Eng. Chem. Proc. Des. Dev. 1976, 15, 439.
- (24) Ranzi, E.; Dente, M.; Costa, A.; Bruzzi V. Thermal decomposition of methane: a mechanistic kinetic scheme. La chimica e l’industria 1988, 70, 1.
- (25) Guéret, C.; Billaud, F. Thermal couling of methane: Influence of hydrogen at 1330°C. Experimental and simulated results. J. Anal. App. Pyrol. 1994, 29, 183.
- (26) Olsvik, O.; Billaud, F. Thermal coupling of methane. A comparison between kinetic model data and experimental data. Thermochim. Acta 1993, 232, 155.
- (27) Liu, F.; Guo, H.; Smallwood, G.J.; Gülder, O.L. The chemical effect of carbon dioxyde as an additive in an ethylene diffusion flame: implications for soot and NOx formation. Combust. Flame 2001, 125, 778.
- (28) Glarborg, P.; Bentzen, L.L.B. Chemical effects of a high CO2 concentration in oxy-fuel combustion of methane. En. Fuels 2008, 22, 291.
- (29) Ranzi, E.; Sogaro, A.; Gaffuri, P.; Pennati, G.; Faravelli T. A wide range modeling study of methane oxidation. Combust. Sci. Techol. 1994, 96, 279.
- (30) Barbé, P.; Battin-Leclerc, F.; Côme G.M. Experimental and modeling study of methane and ethane oxidation between 773 and 1573 K. J. Chim. Phys. 1995, 92, 1666.
- (31) Turbiez, A.; Desgroux, P.; Pauwels, J.F.; Sochet, L.R.; Poitou, S.; Perrin M. GDF-kin® : a new step towards a detailed kinetic mechanism for natural gas combustion modelling. Proc. Intern. Gas Res. Conf. 1998, IV, 371.
- (32) Petersen, E.; Davidson, D.; Hanson R. Kinetics modeling of shock-induced ignition in low-dilution CH4/O2 mixtures at high pressures and intermediate temperatures. Combust. Flame 1999, 117, 272.
- (33) Hidaka, Y.; Sato, K.; Henmi, Y.; Tanaka, H.; Inami K. Shock-tube and modeling study of methane pyrolysis and oxidation, Combust. Flame 1999, 118, 340.
- (34) Coppens, F.H.V.; De Ruyck, J.; Konnov, A.A. The effects of composition on the burning velocity and nitric oxide formation in laminar premixed flames of CH4 + H2 + O2 + N2. Combust. Flame 2007, 149, 409. Detailed reaction mechanism for small hydrocarbons combustion. Release 0.5, (2000), available as Electronic Supplementary Material.
- (35) Smith, G.P.; Golden, D.M.; Frenklach, M.; Moriarty, N.W.; Eiteneer, B.; Goldenberg, M.; Bowman, C.T.; Hanson R.K.; Song, S; Gardiner, Jr., W.C.; Lissianski, V.V.; and Zhiwei Qin http://www.me.berkeley.edu/gri_mech/.
- (36) Jönsson, O. Thermal cracking of tars and hydrocarbons by addition of steam and oxygen in the cracking zone. Fund. Thermochem. Biom. Conv. Overend, R.P.; Milne, T.A.; Mudge, L.K. Elsevier: London, 1985.
- (37) Valin, S.; Castelli, P.; Thiery, S.; Cances, J.; Dufour, A.; Boissonnet, G.; Spindler, B. Upgrading biomass pyrolysis gas by conversion of methane at high temperature: Experiments and modelling. Fuel 2009, 88, 834.
- (38) Morley, C. Gaseq 0.79, A Chemical Equilibrium Program for Windows. http://www.arcl02.dsl.pipex.com/ 2005.
- (39) Fournet, R.; Baugé, J.C.; Battin-Leclerc, F. Experimental and modeling of oxidation of acetylene, propyne, allene and 1,3-butadiene. Int. J. Chem. Kinet. 1999, 31, 305.
- (40) Belmekki, N.; Glaude, P.A.; Da Costa, I.; Fournet, R..; Battin-Leclerc F. Experimental and modeling study of the oxidation of 1-butyne and 2-butyne. Int.J.Chem. Kin. 2002, 34, 172.
- (41) Gueniche, H.A.; Glaude, P.A.; Dayma, G.; Fournet, R.; Battin-Leclerc, F. Rich premixed laminar methane flames doped by light unsaturated hydrocarbons. Combust. Flame 2006, 146, 620.
- (42) Borisov, A.A.; Dragalova, E.V.; Zamanskii, V.M.; Lisyanskii, V.V.; Skachkov G.I. Kinetics and mechanism of methane self-ignition. Khim. Fizika 1982, 4, 536.
- (43) Borisov, A.A.; Zamanskii, V.M.; Konnov, A.A.; Skachkov G.I. Mechanism of high-temperature acetaldehyde oxidation. Sov.J.Chem.Phys. 1990, 6, 748.
- (44) Baulch, D.L.; Cobos, C.J.; Cox, R.A.; Frank, P.; Hayman, G.; Just, T.; Kerr, J.A.; Murrells, T.; Pilling, M.J.; Troe, J.; Walker, R.W.; Warnatz, J. Summary table of evaluated kinetic data for combustion modeling. Combust. Flame 1994, 98, 59.
- (45) Kee, R.J. ; Rupley, F.M. ; Miller J.A. CHEMKIN II, Sandia Laboratories Report, S 89-8009B, 1993.
- (46) Berthelot, M. Sur la Formation des Carbures Pyrogénés. Ann. Chim. Phys. 1869, 16, 143.
- (47) Richter, H.; Howard J.B. Formation of polycyclic aromatic hydrocarbons and their growth to soot—a review of chemical reaction pathways. Prog. Energy Combust. Sci. 2000, 26, 565.
- (48) Roesler, J. F.; Martinot, S.; McEnally, C. S.; Pfefferle, L. D.; Delfau, J.L.; Vovelle C. Investigating the role of methane on the growth of aromatic hydrocarbons and soot in fundamental combustion processes. Combust. Flame 2003, 134, 249.
- (49) Evans, R. J.; Milne, T. A. Molecular characterization of the pyrolysis of biomass. 1. Fundamentals. Energy Fuel 1987, 1, 123.
- (50) Fitzpatrick, E.M.; Jones, J.M.; Pourkashanian, M.; Ross, A.B.; Williams, A.; Bartle K.D. Mechanistic Aspects of Soot Formation from the Combustion of Pine Wood. Energy Fuel 2008, 22, 3771.
fr:Mécanismes et cinétique de la décomposition thermique du méthane dans un gaz de synthèse